Describe the Movement of the Particles in the Air.
The particles move back and forth in the direction of the wave movement Parallel to the wave motion. Indoor air particles come from cooking mold pets and humans.
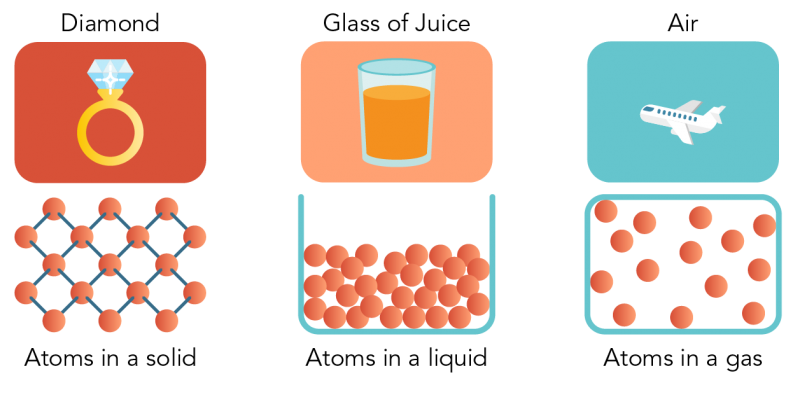
How Are Particles Arranged In The Three States Of Matter Quora
In short there are lots of things in there Ferro says.

. These particles are in constant random motion 3. The particles lose kinetic energy to a point where the forces of attraction are able to hold the particles together tightly. They move in circular paths.
The temperature of the air inside the canister increases. They move with a range of different speeds. For longitudinal waves compare the direction that the particles move to the direction that the wave moves.
Mass of pollutant in 1000 3cm of air nanograms month oxides of nitrogen sulfur dioxide carbon monoxide ozone particulates April 1082 06 13 246 178 May 1216 18 16 232 192 June 1267 16 19 228 200 July 1639 45 2. The speeds of the particles vary but on average they move quicker than they do in. In liquids there is a mild force of attraction between the particles it holds them together but at the same time allow their free movmentwhile in a gasThere is no force of attraction between the particlesso they move randomly Answer link.
The motion of all the particles is predictable. Sound waves traveling through air are a type of wave known as a longitudinal wave. Which two sentences describe the movement of the air particles in the canister.
The particles collide with each other and with the walls of any container in which they are held. They vibrate about a fixed position. It could be dangerous if the temperature of the air inside the canister increased by a large amount.
They take the shape of their container because they have indefinite shape and indefinite volume. The particles in a solid are tightly packed but still moving. The particles of a gas move quickly and have enough energy to spread apart from one another.
Up to 24 cash back Some evidence for the movement of particles in gases is when the perfume gas molecules diffuse into the air when you put it on because the particles from the perfum diffuses with the gas particles around you and it spreads all over your room. Figure 1 shows a diver. Solids have a definite shape and a definite volume.
They vibrate in place. She will have noticed more particles on the outdoor card. Which makes you able to SMELL it.
All matter is composed of tiny particles atoms molecules and ions 2. They move with a range of different speeds. What happens to the movement of the air particles.
-Imagine a box full of air particlesThe particles are forced to move to a point A on the edge of the boxMy question is nowhow can I mathematicly describe the movement of these particles toward point A using one generalised equation. Explain what would be observed when the air temperature is decreased. 101 understand the three states of matter in terms of the arrangement movement and energy of the particles.
The speeds of the particles vary but on average they move quicker than they do in. They move in random directions. This allows the particles to overcome the forces of attraction in a solid which means the regular pattern is broken down and the particles can slide over each other.
2 marks 2 and 4. Ferro is studying particle re-suspension that is the makeup of particles that have landed on indoor surfaces then later detachedby a. See picture Thank you for your time.
The particles move _____ When the temperature of the particles in a gas is increased the particles have more _____energy. Answers The smoke particles will be observed to slow down due to decrease in kinetic energy isaacmuthini answered the question on October 14 2017 at 1317. They vibrate about a fixed position.
Answers and Replies Jul 7 2015 2 mfb. 2 dA gas is put into a closed container. But she will have noticed other particles.
The particles in a gas are moving very quickly in random directions. The amount of energy that the particles lose from these collisions is negligible. The particles in a gas are constantly moving.
The names of the interconversions how they are achieved and the changes in arrangement movement and energy of the particles. The container and the gas inside it are heated. 2 The table shows the mass of air pollutants in nanograms in 1000 cm3 samples of air taken over a four month period.
The particles in a gas are moving very quickly in random directions. They move in random directions. They come from soil pollen forest fires cars trucks and other vehicles.
Particles movement is determined by the energy they have and their relation to other particles. Another evidence for the movement of particles in liquids is when you. A sound wave traveling through air is more or less a macroscopic thing--in order to describe it in the terms you do a wave on a string which by the way is a transverse wave unlike sound waves in air you have to look on a large enough scale that those random molecular motions blur together and things appear smooth and predictable.
The volume of the balloon increases as the. Figure 1 a Which two sentences describe the movement of the air particles in the canister. 2 marks Tick TWO boxes.
102 understand the interconversions between the three states of matter in terms of. 1 Which TWO sentences describe the movement of the air particles in the canister. Gas particles move in a straight-line motion If the temperature of the air in the balloon increases the molecules move faster and have more collisions.
Smoke particle in air when strongly illuminated were observed to describe continuous random haphazard movements. The motion of all the particles is predictable. Describe the particles in a gas.
The particles gain kinetic energy to a point where they vibrate faster. Explain that air has fine particles which cannot be seen without a powerful microscope.
Introduction To The Particle Theory Of Matter Let S Talk Science

Motion Of Particles Flashcards Quizlet

Arrangement Of Particles In Phases Of Matter Comparison Expii
No comments for "Describe the Movement of the Particles in the Air."
Post a Comment